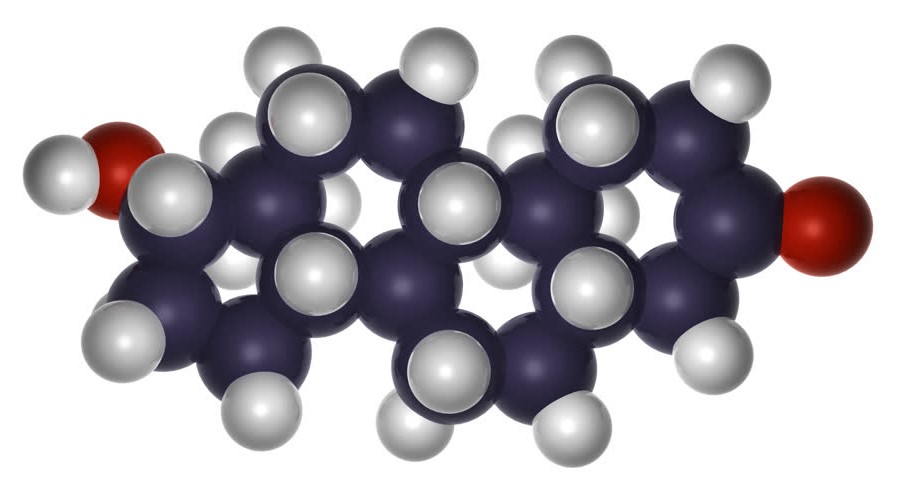
The structure of a steroid molecule is similar to the chemical formula of estrogen and testosterone. Each steroid consists of two carbon atoms and one hydrogen atom, and the methyl group and hydrogen atom in the trans isomer project upwards from the general plane of the molecule. The chemistry of a steriod is a bit complex and is best described by looking at a molecule’s planar structure.
Steroids are metabolic derivatives of terpenes and are named by altering the root structure name. They are defined by the tetracyclic skeleton, consisting of three fused six-membered rings and one five-membered ring. The steroid molecule’s tetracyclic skeleton is made of four linked carbon rings. There are also specific receptors for androgen and estrogen, as well as a broad range of glucocorticoid and mineralcorticoid hormones.
Steroid molecules
Steroid molecules have diverse reactivities. The nature of each functional group affects how easily or how slowly it can form an ester. For example, 3-OH groups are easily reacted with alcohols, while 11b-OH groups require more effort. These properties make steroids highly polar, making them more soluble in oxygen-containing solvents than hydrocarbons. However, the spatial arrangement of atoms also plays a role in the reactivity of a steroid molecule.
The molecular structure of a steroid is complicated. The ring system in steroids is always composed of carbon atoms. A 5-membered ring, in particular, is always connected to a side chain. The side chains can be short, long, or nonexistent. The side chains may be a part of the molecule, or they may not be present at all. When the ring is formed, it is attached to a carbon atom by hydrogen, which is the determining factor for the strength of the ring.
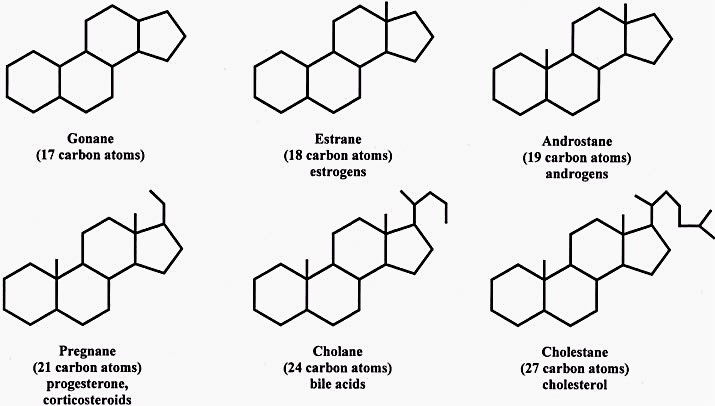
The skeleton of a steroid is composed of seventeen carbon atoms. There are four fused six-membered rings and one five-membered ring. The four carbon atoms are assigned different numbers due to misassignment. The substituents, which are alkyl, are designated with a prefix or suffix. Some steroidal structures are metabolized by breaking the carbon skeleton apart from its fatty acids.
Unlike estrogens and testosterones, steroid molecules can be synthesized synthetically. A steroid molecule can be produced in a laboratory or in a pharmaceutical company. The synthesis of a steroid is a complicated process, but it is crucial to recognize the differences among a steroid molecule and understand its role in the body. Once you know the structure of a steroidal molecule, you can better predict the metabolites in a cellular environment.
Similarly, a steroid molecule can also be synthesized from a ketone. The ring structure is 4 carbons with variable tails and a hydroxyl group attached to the ring. The cis isomer is C3, while the trans isomer is decahydronaphthalene. Its asymmetric carbon atoms are acetate, and ketone is an acetone.
A steroid molecule is made up of hundreds of metabolites, including estrogen, progesterone, testosterone, cholesterol, and guarana. The molecule is characterized by a tetracyclic skeleton, which is a group of four fused rings. A steroid molecule consists of a carbon atom at position three, which is labeled C3 and a hydroxyl group attached to it as R3.
A steroid molecule has four carbons in its skeleton. The tetracyclic skeleton of a steroid molecule is designated by its -ol end. As the most abundant steroid molecule, cholesterol has 27 carbons and a branched aliphatic hydrocarbon unit at C-17. Moreover, cholesterol is the building block of all steroid molecules. In addition to a terpene, other steroid molecules are biosynthesised from it.
In the endocrinological field, testosterone is a molecule with 30 carbons. Its metabolites are found in a variety of organisms, and it is often used to treat or prevent disease. For example, in animals, testosterone is a steroid hormone that causes the growth of the testes and a deepening of the voice. Although it may cause a range of undesirable effects, it can improve the quality of life of the corresponding animal.
Various types of steroid hormones are produced by the body in certain cells and tissues. These molecules contain four interconnected carbon rings. The type of steroid is determined by its polar hydroxyl groups. Several steroid metabolites can be converted into the corresponding metabolite, including cholesterol. This process involves the formation of the precursor of these steroidal hormones. These metabolites are responsible for the growth of various organs and tissues in the body.